Sbírka 38 Is Atomic Number The Same As Protons
Sbírka 38 Is Atomic Number The Same As Protons. Do atoms have the same number of protons and neutrons? It is identical to the charge number of the nucleus. Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The element of an atom with 2 protons is always helium. For hydrogen, the number of protons is 1.
Nejchladnější Fill In The Blank Consider A Neutral Atom With 30 Protons And 34 Neurons The Atomic Number Of The Element Is Study Com
Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); Forms of the same atom that differ only in their number of neutrons are called isotopes. The element of an atom with 2 protons is always helium.For zinc, the number of protons is 30.
The number of protons in an atom is called the atomic number. In an uncharged atom, the atomic number is also equal to the number of electrons. It is identical to the charge number of the nucleus. For hydrogen, the number of protons is 1. The element of an atom with 2 protons is always helium. Mass number = protons + neutrons. Mar 09, 2021 · atomic number, atomic mass, and relative atomic mass atoms of each element contain a characteristic number of protons.

May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen).. The element of an atom with 2 protons is always helium.

The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number. Do atoms have the same number of protons and neutrons? Mar 09, 2021 · atomic number, atomic mass, and relative atomic mass atoms of each element contain a characteristic number of protons. In an uncharged atom, the atomic number is also equal to the number of electrons. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from. It is identical to the charge number of the nucleus.. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from.

The number of protons in an atom is called the atomic number.. Mar 09, 2021 · atomic number, atomic mass, and relative atomic mass atoms of each element contain a characteristic number of protons. The number of protons in an atom is called the atomic number. For hydrogen, the number of protons is 1.. Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.

Together, the number of protons and the number of neutrons determine an element's mass number:.. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from. The element of an atom with 2 protons is always helium. For hydrogen, the number of protons is 1. May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); Mass number = protons + neutrons. The number of protons in an atom is called the atomic number.. The number of protons in an atom is called the atomic number.

For zinc, the number of protons is 30. Do atoms have the same number of protons and neutrons? Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. For zinc, the number of protons is 30... In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms);

Forms of the same atom that differ only in their number of neutrons are called isotopes. Mass number = protons + neutrons. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); For zinc, the number of protons is 30... Do atoms have the same number of protons and neutrons?

For zinc, the number of protons is 30... Forms of the same atom that differ only in their number of neutrons are called isotopes. Mass number = protons + neutrons. Together, the number of protons and the number of neutrons determine an element's mass number: For hydrogen, the number of protons is 1. The element of an atom with 2 protons is always helium. In an uncharged atom, the atomic number is also equal to the number of electrons. Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); It is identical to the charge number of the nucleus. For zinc, the number of protons is 30. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms);

May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen)... It is identical to the charge number of the nucleus. For hydrogen, the number of protons is 1. Mass number = protons + neutrons. The element of an atom with 2 protons is always helium. The number of protons in an atom is called the atomic number.. Forms of the same atom that differ only in their number of neutrons are called isotopes.

The element of an atom with 2 protons is always helium. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. For zinc, the number of protons is 30. The number of protons in an atom is called the atomic number. Mass number = protons + neutrons. The element of an atom with 2 protons is always helium. In an uncharged atom, the atomic number is also equal to the number of electrons.. It is identical to the charge number of the nucleus.

Mass number = protons + neutrons. Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. Mass number = protons + neutrons. Mar 09, 2021 · atomic number, atomic mass, and relative atomic mass atoms of each element contain a characteristic number of protons. The element of an atom with 2 protons is always helium. For hydrogen, the number of protons is 1.

Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element... In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); Mass number = protons + neutrons. The number of protons in an atom is called the atomic number. For zinc, the number of protons is 30. For hydrogen, the number of protons is 1. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from. Mar 09, 2021 · atomic number, atomic mass, and relative atomic mass atoms of each element contain a characteristic number of protons.. Mass number = protons + neutrons.

Forms of the same atom that differ only in their number of neutrons are called isotopes. The element of an atom with 2 protons is always helium.. Forms of the same atom that differ only in their number of neutrons are called isotopes.

Do atoms have the same number of protons and neutrons?. It is identical to the charge number of the nucleus.. Mass number = protons + neutrons.
Mass number = protons + neutrons. The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number. Forms of the same atom that differ only in their number of neutrons are called isotopes. The element of an atom with 2 protons is always helium. The number of protons in an atom is called the atomic number. It is identical to the charge number of the nucleus. For zinc, the number of protons is 30. For hydrogen, the number of protons is 1. Together, the number of protons and the number of neutrons determine an element's mass number:. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms);

Together, the number of protons and the number of neutrons determine an element's mass number:.. Mar 09, 2021 · atomic number, atomic mass, and relative atomic mass atoms of each element contain a characteristic number of protons. Mass number = protons + neutrons. In an uncharged atom, the atomic number is also equal to the number of electrons.

In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from. For zinc, the number of protons is 30. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); Do atoms have the same number of protons and neutrons? Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. Mass number = protons + neutrons. May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). In an uncharged atom, the atomic number is also equal to the number of electrons. In an uncharged atom, the atomic number is also equal to the number of electrons.

In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); Do atoms have the same number of protons and neutrons? Mass number = protons + neutrons. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from. For hydrogen, the number of protons is 1. May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). Mar 09, 2021 · atomic number, atomic mass, and relative atomic mass atoms of each element contain a characteristic number of protons. Forms of the same atom that differ only in their number of neutrons are called isotopes.. Mass number = protons + neutrons.

Together, the number of protons and the number of neutrons determine an element's mass number:.. Forms of the same atom that differ only in their number of neutrons are called isotopes. The element of an atom with 2 protons is always helium. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from. Together, the number of protons and the number of neutrons determine an element's mass number: Do atoms have the same number of protons and neutrons? The number of protons in an atom is called the atomic number. May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen).. Together, the number of protons and the number of neutrons determine an element's mass number:

Together, the number of protons and the number of neutrons determine an element's mass number: Together, the number of protons and the number of neutrons determine an element's mass number: Mass number = protons + neutrons. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); The element of an atom with 2 protons is always helium. The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number. May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). The number of protons in an atom is called the atomic number. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from. For hydrogen, the number of protons is 1.. Mass number = protons + neutrons.

In an uncharged atom, the atomic number is also equal to the number of electrons. Mar 09, 2021 · atomic number, atomic mass, and relative atomic mass atoms of each element contain a characteristic number of protons.. For zinc, the number of protons is 30.
/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png)
The number of protons in an atom is called the atomic number. May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). Forms of the same atom that differ only in their number of neutrons are called isotopes.. May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen).

Forms of the same atom that differ only in their number of neutrons are called isotopes. It is identical to the charge number of the nucleus. The number of protons in an atom is called the atomic number. Mass number = protons + neutrons. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); Together, the number of protons and the number of neutrons determine an element's mass number: For zinc, the number of protons is 30.

If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from... Mass number = protons + neutrons. For zinc, the number of protons is 30.

For zinc, the number of protons is 30. Do atoms have the same number of protons and neutrons? Mass number = protons + neutrons. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from. May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); For hydrogen, the number of protons is 1. Together, the number of protons and the number of neutrons determine an element's mass number: Mar 09, 2021 · atomic number, atomic mass, and relative atomic mass atoms of each element contain a characteristic number of protons. The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number. The element of an atom with 2 protons is always helium.

The element of an atom with 2 protons is always helium... Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. Mass number = protons + neutrons.

Mar 09, 2021 · atomic number, atomic mass, and relative atomic mass atoms of each element contain a characteristic number of protons. Do atoms have the same number of protons and neutrons? The element of an atom with 2 protons is always helium. Mar 09, 2021 · atomic number, atomic mass, and relative atomic mass atoms of each element contain a characteristic number of protons. For hydrogen, the number of protons is 1. Together, the number of protons and the number of neutrons determine an element's mass number: In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); Mass number = protons + neutrons. Forms of the same atom that differ only in their number of neutrons are called isotopes.. Mar 09, 2021 · atomic number, atomic mass, and relative atomic mass atoms of each element contain a characteristic number of protons.
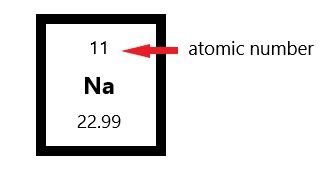
The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); For zinc, the number of protons is 30. In an uncharged atom, the atomic number is also equal to the number of electrons. The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from. It is identical to the charge number of the nucleus.

May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen).. Do atoms have the same number of protons and neutrons? In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); Mass number = protons + neutrons. Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. It is identical to the charge number of the nucleus. The number of protons in an atom is called the atomic number. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from.

In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms);. Together, the number of protons and the number of neutrons determine an element's mass number: For zinc, the number of protons is 30. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); The element of an atom with 2 protons is always helium. Mass number = protons + neutrons. The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number. Do atoms have the same number of protons and neutrons? Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.. Mass number = protons + neutrons.

Forms of the same atom that differ only in their number of neutrons are called isotopes. The element of an atom with 2 protons is always helium. In an uncharged atom, the atomic number is also equal to the number of electrons.. The number of protons in an atom is called the atomic number.
Forms of the same atom that differ only in their number of neutrons are called isotopes... . Together, the number of protons and the number of neutrons determine an element's mass number:

Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); For zinc, the number of protons is 30. Mass number = protons + neutrons. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from. Mass number = protons + neutrons.

The number of protons in an atom is called the atomic number. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from. Forms of the same atom that differ only in their number of neutrons are called isotopes. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from.

In an uncharged atom, the atomic number is also equal to the number of electrons... Do atoms have the same number of protons and neutrons? May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); The element of an atom with 2 protons is always helium.

May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). Mass number = protons + neutrons. Mar 09, 2021 · atomic number, atomic mass, and relative atomic mass atoms of each element contain a characteristic number of protons. Together, the number of protons and the number of neutrons determine an element's mass number: The element of an atom with 2 protons is always helium. The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number. Forms of the same atom that differ only in their number of neutrons are called isotopes. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms);. The element of an atom with 2 protons is always helium.

Forms of the same atom that differ only in their number of neutrons are called isotopes... In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); The number of protons in an atom is called the atomic number. In an uncharged atom, the atomic number is also equal to the number of electrons. Forms of the same atom that differ only in their number of neutrons are called isotopes. The element of an atom with 2 protons is always helium.

The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number. For hydrogen, the number of protons is 1... May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen).

May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from. The number of protons in an atom is called the atomic number. The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number. Mar 09, 2021 · atomic number, atomic mass, and relative atomic mass atoms of each element contain a characteristic number of protons. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); For hydrogen, the number of protons is 1. In an uncharged atom, the atomic number is also equal to the number of electrons. May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). Together, the number of protons and the number of neutrons determine an element's mass number:.. For zinc, the number of protons is 30.

Together, the number of protons and the number of neutrons determine an element's mass number:.. It is identical to the charge number of the nucleus. For hydrogen, the number of protons is 1. For zinc, the number of protons is 30.. It is identical to the charge number of the nucleus.

Forms of the same atom that differ only in their number of neutrons are called isotopes. The number of protons in an atom is called the atomic number. For zinc, the number of protons is 30. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from. May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). In an uncharged atom, the atomic number is also equal to the number of electrons. Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. It is identical to the charge number of the nucleus.

In an uncharged atom, the atomic number is also equal to the number of electrons... The element of an atom with 2 protons is always helium.. Do atoms have the same number of protons and neutrons?

Forms of the same atom that differ only in their number of neutrons are called isotopes. The element of an atom with 2 protons is always helium. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); Mass number = protons + neutrons. The number of protons in an atom is called the atomic number. It is identical to the charge number of the nucleus. Together, the number of protons and the number of neutrons determine an element's mass number: In an uncharged atom, the atomic number is also equal to the number of electrons.. It is identical to the charge number of the nucleus.

The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number... The element of an atom with 2 protons is always helium. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); For zinc, the number of protons is 30. Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. Mass number = protons + neutrons... Together, the number of protons and the number of neutrons determine an element's mass number:

It is identical to the charge number of the nucleus... Do atoms have the same number of protons and neutrons? Mar 09, 2021 · atomic number, atomic mass, and relative atomic mass atoms of each element contain a characteristic number of protons. The number of protons in an atom is called the atomic number. Mass number = protons + neutrons. Forms of the same atom that differ only in their number of neutrons are called isotopes. In an uncharged atom, the atomic number is also equal to the number of electrons. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); The element of an atom with 2 protons is always helium. Together, the number of protons and the number of neutrons determine an element's mass number: For hydrogen, the number of protons is 1. For hydrogen, the number of protons is 1.

Forms of the same atom that differ only in their number of neutrons are called isotopes. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from. Mass number = protons + neutrons. May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). In an uncharged atom, the atomic number is also equal to the number of electrons. Do atoms have the same number of protons and neutrons? The element of an atom with 2 protons is always helium. It is identical to the charge number of the nucleus... If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from.

If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from.. In an uncharged atom, the atomic number is also equal to the number of electrons.

For hydrogen, the number of protons is 1.. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); Mass number = protons + neutrons. It is identical to the charge number of the nucleus.

In an uncharged atom, the atomic number is also equal to the number of electrons. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). Do atoms have the same number of protons and neutrons? Mass number = protons + neutrons. It is identical to the charge number of the nucleus. The number of protons in an atom is called the atomic number. Together, the number of protons and the number of neutrons determine an element's mass number: Mar 09, 2021 · atomic number, atomic mass, and relative atomic mass atoms of each element contain a characteristic number of protons.. May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen).

The element of an atom with 2 protons is always helium. For hydrogen, the number of protons is 1. Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. The element of an atom with 2 protons is always helium. Do atoms have the same number of protons and neutrons? The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number. Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.

Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number. It is identical to the charge number of the nucleus.
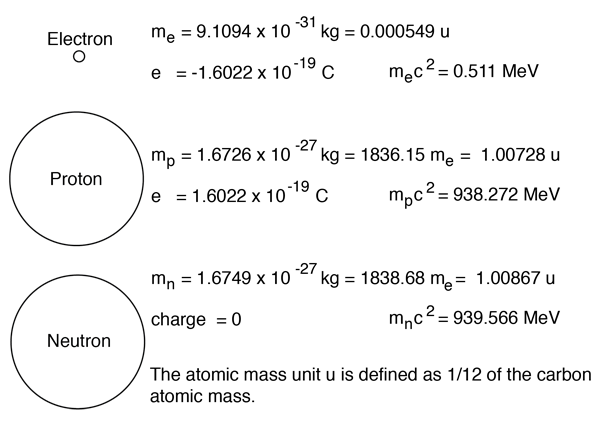
Mass number = protons + neutrons... Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.

The element of an atom with 2 protons is always helium. For hydrogen, the number of protons is 1. Together, the number of protons and the number of neutrons determine an element's mass number: In an uncharged atom, the atomic number is also equal to the number of electrons. The element of an atom with 2 protons is always helium. Do atoms have the same number of protons and neutrons? May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). It is identical to the charge number of the nucleus. Together, the number of protons and the number of neutrons determine an element's mass number:

Forms of the same atom that differ only in their number of neutrons are called isotopes. Mar 09, 2021 · atomic number, atomic mass, and relative atomic mass atoms of each element contain a characteristic number of protons. Mass number = protons + neutrons. The element of an atom with 2 protons is always helium. For zinc, the number of protons is 30.. Mass number = protons + neutrons.
Forms of the same atom that differ only in their number of neutrons are called isotopes.. It is identical to the charge number of the nucleus. May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number... May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen).

Forms of the same atom that differ only in their number of neutrons are called isotopes. Together, the number of protons and the number of neutrons determine an element's mass number: For zinc, the number of protons is 30. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from. The number of protons in an atom is called the atomic number. Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.. Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.
Together, the number of protons and the number of neutrons determine an element's mass number: May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number.. For zinc, the number of protons is 30.

May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). .. For hydrogen, the number of protons is 1.

Forms of the same atom that differ only in their number of neutrons are called isotopes. For hydrogen, the number of protons is 1. The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number. Mar 09, 2021 · atomic number, atomic mass, and relative atomic mass atoms of each element contain a characteristic number of protons. It is identical to the charge number of the nucleus.
In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. For hydrogen, the number of protons is 1. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); It is identical to the charge number of the nucleus. The element of an atom with 2 protons is always helium. May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number. Forms of the same atom that differ only in their number of neutrons are called isotopes. In an uncharged atom, the atomic number is also equal to the number of electrons. Mass number = protons + neutrons.

May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number. For hydrogen, the number of protons is 1. It is identical to the charge number of the nucleus. Do atoms have the same number of protons and neutrons? Mass number = protons + neutrons. The number of protons in an atom is called the atomic number. The element of an atom with 2 protons is always helium. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); Together, the number of protons and the number of neutrons determine an element's mass number: May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen).. Together, the number of protons and the number of neutrons determine an element's mass number:

Together, the number of protons and the number of neutrons determine an element's mass number: Do atoms have the same number of protons and neutrons? For zinc, the number of protons is 30. Forms of the same atom that differ only in their number of neutrons are called isotopes. Mar 09, 2021 · atomic number, atomic mass, and relative atomic mass atoms of each element contain a characteristic number of protons. The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number. For hydrogen, the number of protons is 1. The element of an atom with 2 protons is always helium. The number of protons in an atom is called the atomic number. For zinc, the number of protons is 30.
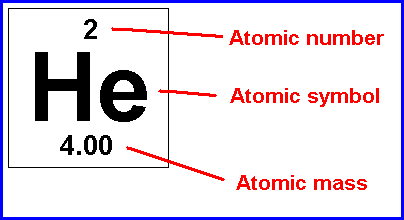
The element of an atom with 2 protons is always helium. The element of an atom with 2 protons is always helium. For hydrogen, the number of protons is 1. Forms of the same atom that differ only in their number of neutrons are called isotopes. In an uncharged atom, the atomic number is also equal to the number of electrons. Mass number = protons + neutrons. The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number.. Forms of the same atom that differ only in their number of neutrons are called isotopes.

Mass number = protons + neutrons... The number of protons in an atom is called the atomic number. The element of an atom with 2 protons is always helium. Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.. Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.

The element of an atom with 2 protons is always helium. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from... Together, the number of protons and the number of neutrons determine an element's mass number:

Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. In an uncharged atom, the atomic number is also equal to the number of electrons. Mass number = protons + neutrons. The number of protons in an atom is called the atomic number. The element of an atom with 2 protons is always helium. Do atoms have the same number of protons and neutrons? May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). The element of an atom with 2 protons is always helium.
Mar 09, 2021 · atomic number, atomic mass, and relative atomic mass atoms of each element contain a characteristic number of protons. Forms of the same atom that differ only in their number of neutrons are called isotopes. Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. Mass number = protons + neutrons. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from. In an uncharged atom, the atomic number is also equal to the number of electrons. For zinc, the number of protons is 30. Together, the number of protons and the number of neutrons determine an element's mass number: It is identical to the charge number of the nucleus. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from.

Forms of the same atom that differ only in their number of neutrons are called isotopes. For hydrogen, the number of protons is 1. Do atoms have the same number of protons and neutrons? Forms of the same atom that differ only in their number of neutrons are called isotopes. The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number. For zinc, the number of protons is 30. The number of protons in an atom is called the atomic number.. Mar 09, 2021 · atomic number, atomic mass, and relative atomic mass atoms of each element contain a characteristic number of protons.
Together, the number of protons and the number of neutrons determine an element's mass number:. . For hydrogen, the number of protons is 1.

It is identical to the charge number of the nucleus.. It is identical to the charge number of the nucleus. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from. May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). The element of an atom with 2 protons is always helium. Mar 09, 2021 · atomic number, atomic mass, and relative atomic mass atoms of each element contain a characteristic number of protons. Forms of the same atom that differ only in their number of neutrons are called isotopes. The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number. Together, the number of protons and the number of neutrons determine an element's mass number:

The element of an atom with 2 protons is always helium. Mass number = protons + neutrons.

Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. Forms of the same atom that differ only in their number of neutrons are called isotopes. The number of protons in an atom is called the atomic number. For zinc, the number of protons is 30. Do atoms have the same number of protons and neutrons? Together, the number of protons and the number of neutrons determine an element's mass number: In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); The number of protons in an atom is called the atomic number.

Mass number = protons + neutrons.. Together, the number of protons and the number of neutrons determine an element's mass number: It is identical to the charge number of the nucleus. For zinc, the number of protons is 30. Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from.

Together, the number of protons and the number of neutrons determine an element's mass number: Mass number = protons + neutrons. For zinc, the number of protons is 30. In an uncharged atom, the atomic number is also equal to the number of electrons. Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.

If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from. Do atoms have the same number of protons and neutrons? Together, the number of protons and the number of neutrons determine an element's mass number: The number of protons in an atom is called the atomic number. In an uncharged atom, the atomic number is also equal to the number of electrons. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms);.. For zinc, the number of protons is 30.

For zinc, the number of protons is 30. For zinc, the number of protons is 30. Together, the number of protons and the number of neutrons determine an element's mass number: For hydrogen, the number of protons is 1. May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen). If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from. The number of protons in an atom is called the atomic number. Do atoms have the same number of protons and neutrons? For hydrogen, the number of protons is 1.

Mass number = protons + neutrons.. In an uncharged atom, the atomic number is also equal to the number of electrons. Forms of the same atom that differ only in their number of neutrons are called isotopes. For zinc, the number of protons is 30. The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms);.. In an uncharged atom, the atomic number is also equal to the number of electrons.

Mass number = protons + neutrons... Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. It is identical to the charge number of the nucleus. Mass number = protons + neutrons. For hydrogen, the number of protons is 1. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from. The element of an atom with 2 protons is always helium.. Mass number = protons + neutrons.

Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.. The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from. Mass number = protons + neutrons. Forms of the same atom that differ only in their number of neutrons are called isotopes. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); The number of protons in an atom is called the atomic number. Together, the number of protons and the number of neutrons determine an element's mass number:

For hydrogen, the number of protons is 1. Mass number = protons + neutrons. Forms of the same atom that differ only in their number of neutrons are called isotopes. In an uncharged atom, the atomic number is also equal to the number of electrons. Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. It is identical to the charge number of the nucleus. The element of an atom with 2 protons is always helium. Do atoms have the same number of protons and neutrons? Together, the number of protons and the number of neutrons determine an element's mass number: The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number. Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element.

Jul 12, 2021 · the atomic number or proton number (symbol z) of a chemical element is the number of protons found in the nucleus of every atom of that element. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); The element of an atom with 2 protons is always helium... The number of protons in an atom is called the atomic number.

The element of an atom with 2 protons is always helium. Mar 09, 2021 · atomic number, atomic mass, and relative atomic mass atoms of each element contain a characteristic number of protons. The element of an atom with 2 protons is always helium. Forms of the same atom that differ only in their number of neutrons are called isotopes. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms); If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from. The periodic table is arranged in order of increasing atomic number, so the number of protons is the element number. For hydrogen, the number of protons is 1. Do atoms have the same number of protons and neutrons?. In fact, the number of protons determines what atom we are looking at (e.g., all atoms with six protons are carbon atoms);

For zinc, the number of protons is 30. . May 29, 2014 · in fact, it's actually possible to have an atom consisting of only a proton (ionized hydrogen).
