Seznamy Atom Diagram With Subatomic Particles
Seznamy Atom Diagram With Subatomic Particles. These particles were electrically neutral and called neutrons. With the discovery of protons, neutrons, and electrons, physicists could put forth a … The central part of an atom containing protons and neutrons match each item with the correct statement: Download my free ebook 10 secrets to acing organic chemistry here: The numbers of subatomic particles in an atom …
Tady Draw A Bohr Model Of Beryllium Draw A Bohr Model Of Chlorine Activity Ppt Download
Today we know over 200. These particles were electrically neutral and called neutrons. Use colored candy to represent subatomic particles and make a model of an atom (bohr model). These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u.Use colored candy to represent subatomic particles and make a model of an atom (bohr model).
Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks. The diagram on the right is traditionally used to represent an atom, as proposed by niels bohr in 1913.it was nicknamed the planetary model, where electrons (the planets) orbit the nucleus (the star).in this model, the electrons can only be at certain energy levels.this model successfully explained the nature of the light emitted by some elements excited by a source of heat and the concepts of. A subatomic particle with no charge s. Total of protons and neutrons in the. Download my free ebook 10 secrets to acing organic chemistry here: These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u.

The numbers of subatomic particles in an atom … . Today we know over 200.

These particles were electrically neutral and called neutrons.. Download my free ebook 10 secrets to acing organic chemistry here: These particles were electrically neutral and called neutrons. • a periodic table (see below) A negatively charged subatomic particle g. Atoms with the same number of protons but different numbers of neutrons 2. Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks. A positively charged subatomic particle 3. Today we know over 200. The central part of an atom containing protons and neutrons match each item with the correct statement: These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u.. Today we know over 200.

Download my free ebook 10 secrets to acing organic chemistry here: Total of protons and neutrons in the. Use colored candy to represent subatomic particles and make a model of an atom (bohr model). The diagram on the right is traditionally used to represent an atom, as proposed by niels bohr in 1913.it was nicknamed the planetary model, where electrons (the planets) orbit the nucleus (the star).in this model, the electrons can only be at certain energy levels.this model successfully explained the nature of the light emitted by some elements excited by a source of heat and the concepts of. M and m® model of the atom edible subatomic particles by eric muller introduction: The central part of an atom containing protons and neutrons match each item with the correct statement: These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. Atoms with the same number of protons but different numbers of neutrons 2. A subatomic particle with no charge s.. A subatomic particle with no charge s.
Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks. Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks. M and m® model of the atom edible subatomic particles by eric muller introduction: A negatively charged subatomic particle g. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. • a periodic table (see below) Download my free ebook 10 secrets to acing organic chemistry here: Today we know over 200. Total of protons and neutrons in the. The diagram on the right is traditionally used to represent an atom, as proposed by niels bohr in 1913.it was nicknamed the planetary model, where electrons (the planets) orbit the nucleus (the star).in this model, the electrons can only be at certain energy levels.this model successfully explained the nature of the light emitted by some elements excited by a source of heat and the concepts of. Use colored candy to represent subatomic particles and make a model of an atom (bohr model)... Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks.

The diagram on the right is traditionally used to represent an atom, as proposed by niels bohr in 1913.it was nicknamed the planetary model, where electrons (the planets) orbit the nucleus (the star).in this model, the electrons can only be at certain energy levels.this model successfully explained the nature of the light emitted by some elements excited by a source of heat and the concepts of... Atoms with the same number of protons but different numbers of neutrons 2. A subatomic particle with no charge s. The central part of an atom containing protons and neutrons match each item with the correct statement: Today we know over 200. These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. • a periodic table (see below) Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks... Total of protons and neutrons in the.

Atoms with the same number of protons but different numbers of neutrons 2. • a periodic table (see below) A subatomic particle with no charge s. Total of protons and neutrons in the. A negatively charged subatomic particle g. Download my free ebook 10 secrets to acing organic chemistry here:

Use colored candy to represent subatomic particles and make a model of an atom (bohr model). A negatively charged subatomic particle g. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. The diagram on the right is traditionally used to represent an atom, as proposed by niels bohr in 1913.it was nicknamed the planetary model, where electrons (the planets) orbit the nucleus (the star).in this model, the electrons can only be at certain energy levels.this model successfully explained the nature of the light emitted by some elements excited by a source of heat and the concepts of. A subatomic particle with no charge s. With the discovery of protons, neutrons, and electrons, physicists could put forth a … A positively charged subatomic particle 3. Total of protons and neutrons in the. The numbers of subatomic particles in an atom … Atoms with the same number of protons but different numbers of neutrons 2... A negatively charged subatomic particle g.

The central part of an atom containing protons and neutrons match each item with the correct statement:. The central part of an atom containing protons and neutrons match each item with the correct statement: With the discovery of protons, neutrons, and electrons, physicists could put forth a … Today we know over 200.. The diagram on the right is traditionally used to represent an atom, as proposed by niels bohr in 1913.it was nicknamed the planetary model, where electrons (the planets) orbit the nucleus (the star).in this model, the electrons can only be at certain energy levels.this model successfully explained the nature of the light emitted by some elements excited by a source of heat and the concepts of.

Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks.. With the discovery of protons, neutrons, and electrons, physicists could put forth a … A negatively charged subatomic particle g. Atoms with the same number of protons but different numbers of neutrons 2. Total of protons and neutrons in the. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Today we know over 200. Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks.. Download my free ebook 10 secrets to acing organic chemistry here:

With the discovery of protons, neutrons, and electrons, physicists could put forth a …. These particles were electrically neutral and called neutrons... Use colored candy to represent subatomic particles and make a model of an atom (bohr model).

The central part of an atom containing protons and neutrons match each item with the correct statement: These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. With the discovery of protons, neutrons, and electrons, physicists could put forth a … M and m® model of the atom edible subatomic particles by eric muller introduction: Atoms with the same number of protons but different numbers of neutrons 2. Today we know over 200. A positively charged subatomic particle 3. These particles were electrically neutral and called neutrons. Download my free ebook 10 secrets to acing organic chemistry here: The diagram on the right is traditionally used to represent an atom, as proposed by niels bohr in 1913.it was nicknamed the planetary model, where electrons (the planets) orbit the nucleus (the star).in this model, the electrons can only be at certain energy levels.this model successfully explained the nature of the light emitted by some elements excited by a source of heat and the concepts of. The numbers of subatomic particles in an atom …. A positively charged subatomic particle 3.

A subatomic particle with no charge s. The numbers of subatomic particles in an atom … The diagram on the right is traditionally used to represent an atom, as proposed by niels bohr in 1913.it was nicknamed the planetary model, where electrons (the planets) orbit the nucleus (the star).in this model, the electrons can only be at certain energy levels.this model successfully explained the nature of the light emitted by some elements excited by a source of heat and the concepts of. With the discovery of protons, neutrons, and electrons, physicists could put forth a … Today we know over 200. A subatomic particle with no charge s. The central part of an atom containing protons and neutrons match each item with the correct statement: These particles were electrically neutral and called neutrons. A positively charged subatomic particle 3. M and m® model of the atom edible subatomic particles by eric muller introduction:

Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom... A subatomic particle with no charge s. The central part of an atom containing protons and neutrons match each item with the correct statement: Today we know over 200. Total of protons and neutrons in the. Atoms with the same number of protons but different numbers of neutrons 2. These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u.. These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u.

These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u.. Use colored candy to represent subatomic particles and make a model of an atom (bohr model). These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. The central part of an atom containing protons and neutrons match each item with the correct statement: With the discovery of protons, neutrons, and electrons, physicists could put forth a … Atoms with the same number of protons but different numbers of neutrons 2. A negatively charged subatomic particle g. Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks. Total of protons and neutrons in the. Today we know over 200. Download my free ebook 10 secrets to acing organic chemistry here:. A subatomic particle with no charge s.

Total of protons and neutrons in the. . Use colored candy to represent subatomic particles and make a model of an atom (bohr model).

• a periodic table (see below). Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks. A negatively charged subatomic particle g. The numbers of subatomic particles in an atom … M and m® model of the atom edible subatomic particles by eric muller introduction:. Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks.
These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. Total of protons and neutrons in the. Atoms with the same number of protons but different numbers of neutrons 2. M and m® model of the atom edible subatomic particles by eric muller introduction: Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. The numbers of subatomic particles in an atom … Download my free ebook 10 secrets to acing organic chemistry here: The central part of an atom containing protons and neutrons match each item with the correct statement:

Today we know over 200. These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks. Total of protons and neutrons in the.

These particles were electrically neutral and called neutrons.. A positively charged subatomic particle 3. • a periodic table (see below) Use colored candy to represent subatomic particles and make a model of an atom (bohr model). Atoms with the same number of protons but different numbers of neutrons 2. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. Total of protons and neutrons in the. The diagram on the right is traditionally used to represent an atom, as proposed by niels bohr in 1913.it was nicknamed the planetary model, where electrons (the planets) orbit the nucleus (the star).in this model, the electrons can only be at certain energy levels.this model successfully explained the nature of the light emitted by some elements excited by a source of heat and the concepts of... • a periodic table (see below)

A subatomic particle with no charge s.. The numbers of subatomic particles in an atom … • a periodic table (see below) Atoms with the same number of protons but different numbers of neutrons 2. With the discovery of protons, neutrons, and electrons, physicists could put forth a … The central part of an atom containing protons and neutrons match each item with the correct statement: Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. A subatomic particle with no charge s. Total of protons and neutrons in the. M and m® model of the atom edible subatomic particles by eric muller introduction: A negatively charged subatomic particle g.. A positively charged subatomic particle 3.

Total of protons and neutrons in the. Atoms with the same number of protons but different numbers of neutrons 2.. Download my free ebook 10 secrets to acing organic chemistry here:

The central part of an atom containing protons and neutrons match each item with the correct statement: These particles were electrically neutral and called neutrons. The diagram on the right is traditionally used to represent an atom, as proposed by niels bohr in 1913.it was nicknamed the planetary model, where electrons (the planets) orbit the nucleus (the star).in this model, the electrons can only be at certain energy levels.this model successfully explained the nature of the light emitted by some elements excited by a source of heat and the concepts of. A negatively charged subatomic particle g. The central part of an atom containing protons and neutrons match each item with the correct statement: These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. A subatomic particle with no charge s. Today we know over 200.. Today we know over 200.

The diagram on the right is traditionally used to represent an atom, as proposed by niels bohr in 1913.it was nicknamed the planetary model, where electrons (the planets) orbit the nucleus (the star).in this model, the electrons can only be at certain energy levels.this model successfully explained the nature of the light emitted by some elements excited by a source of heat and the concepts of. M and m® model of the atom edible subatomic particles by eric muller introduction: Download my free ebook 10 secrets to acing organic chemistry here: A subatomic particle with no charge s. The numbers of subatomic particles in an atom … The diagram on the right is traditionally used to represent an atom, as proposed by niels bohr in 1913.it was nicknamed the planetary model, where electrons (the planets) orbit the nucleus (the star).in this model, the electrons can only be at certain energy levels.this model successfully explained the nature of the light emitted by some elements excited by a source of heat and the concepts of. Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks. The central part of an atom containing protons and neutrons match each item with the correct statement: Use colored candy to represent subatomic particles and make a model of an atom (bohr model). Atoms with the same number of protons but different numbers of neutrons 2.. Use colored candy to represent subatomic particles and make a model of an atom (bohr model).
Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.. M and m® model of the atom edible subatomic particles by eric muller introduction: The diagram on the right is traditionally used to represent an atom, as proposed by niels bohr in 1913.it was nicknamed the planetary model, where electrons (the planets) orbit the nucleus (the star).in this model, the electrons can only be at certain energy levels.this model successfully explained the nature of the light emitted by some elements excited by a source of heat and the concepts of. The numbers of subatomic particles in an atom … • a periodic table (see below) Total of protons and neutrons in the... Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks.

A positively charged subatomic particle 3... . A negatively charged subatomic particle g.

These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. Atoms with the same number of protons but different numbers of neutrons 2. Total of protons and neutrons in the. The numbers of subatomic particles in an atom … A subatomic particle with no charge s. With the discovery of protons, neutrons, and electrons, physicists could put forth a … M and m® model of the atom edible subatomic particles by eric muller introduction: A positively charged subatomic particle 3. The diagram on the right is traditionally used to represent an atom, as proposed by niels bohr in 1913.it was nicknamed the planetary model, where electrons (the planets) orbit the nucleus (the star).in this model, the electrons can only be at certain energy levels.this model successfully explained the nature of the light emitted by some elements excited by a source of heat and the concepts of. With the discovery of protons, neutrons, and electrons, physicists could put forth a …

These particles were electrically neutral and called neutrons. Use colored candy to represent subatomic particles and make a model of an atom (bohr model).. These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u.

With the discovery of protons, neutrons, and electrons, physicists could put forth a … Today we know over 200. A subatomic particle with no charge s. Total of protons and neutrons in the.

These particles were electrically neutral and called neutrons... Download my free ebook 10 secrets to acing organic chemistry here: The numbers of subatomic particles in an atom ….. M and m® model of the atom edible subatomic particles by eric muller introduction:

Today we know over 200. • a periodic table (see below) Use colored candy to represent subatomic particles and make a model of an atom (bohr model). M and m® model of the atom edible subatomic particles by eric muller introduction: Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks.. Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks.

The central part of an atom containing protons and neutrons match each item with the correct statement: Today we know over 200. These particles were electrically neutral and called neutrons. The numbers of subatomic particles in an atom … With the discovery of protons, neutrons, and electrons, physicists could put forth a …. Download my free ebook 10 secrets to acing organic chemistry here:
/atom-drawn-by-scientist-or-student-155287893-584ee6855f9b58a8cd2fc8f1.jpg)
Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks. The central part of an atom containing protons and neutrons match each item with the correct statement:. Download my free ebook 10 secrets to acing organic chemistry here:
A subatomic particle with no charge s. The diagram on the right is traditionally used to represent an atom, as proposed by niels bohr in 1913.it was nicknamed the planetary model, where electrons (the planets) orbit the nucleus (the star).in this model, the electrons can only be at certain energy levels.this model successfully explained the nature of the light emitted by some elements excited by a source of heat and the concepts of. • a periodic table (see below) With the discovery of protons, neutrons, and electrons, physicists could put forth a … The central part of an atom containing protons and neutrons match each item with the correct statement:. Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks.

Use colored candy to represent subatomic particles and make a model of an atom (bohr model).. The numbers of subatomic particles in an atom … • a periodic table (see below) With the discovery of protons, neutrons, and electrons, physicists could put forth a … M and m® model of the atom edible subatomic particles by eric muller introduction: The diagram on the right is traditionally used to represent an atom, as proposed by niels bohr in 1913.it was nicknamed the planetary model, where electrons (the planets) orbit the nucleus (the star).in this model, the electrons can only be at certain energy levels.this model successfully explained the nature of the light emitted by some elements excited by a source of heat and the concepts of.

A subatomic particle with no charge s. .. Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks.

Total of protons and neutrons in the. Today we know over 200. With the discovery of protons, neutrons, and electrons, physicists could put forth a … The diagram on the right is traditionally used to represent an atom, as proposed by niels bohr in 1913.it was nicknamed the planetary model, where electrons (the planets) orbit the nucleus (the star).in this model, the electrons can only be at certain energy levels.this model successfully explained the nature of the light emitted by some elements excited by a source of heat and the concepts of. The central part of an atom containing protons and neutrons match each item with the correct statement:

The central part of an atom containing protons and neutrons match each item with the correct statement: M and m® model of the atom edible subatomic particles by eric muller introduction: These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. A positively charged subatomic particle 3. With the discovery of protons, neutrons, and electrons, physicists could put forth a ….. Atoms with the same number of protons but different numbers of neutrons 2.

Total of protons and neutrons in the.. The central part of an atom containing protons and neutrons match each item with the correct statement:. M and m® model of the atom edible subatomic particles by eric muller introduction:

Download my free ebook 10 secrets to acing organic chemistry here:. . A positively charged subatomic particle 3.

A positively charged subatomic particle 3. Today we know over 200. M and m® model of the atom edible subatomic particles by eric muller introduction: Atoms with the same number of protons but different numbers of neutrons 2. A positively charged subatomic particle 3. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom. With the discovery of protons, neutrons, and electrons, physicists could put forth a … The numbers of subatomic particles in an atom … The diagram on the right is traditionally used to represent an atom, as proposed by niels bohr in 1913.it was nicknamed the planetary model, where electrons (the planets) orbit the nucleus (the star).in this model, the electrons can only be at certain energy levels.this model successfully explained the nature of the light emitted by some elements excited by a source of heat and the concepts of. Total of protons and neutrons in the. A negatively charged subatomic particle g.. Atoms with the same number of protons but different numbers of neutrons 2.

A positively charged subatomic particle 3. M and m® model of the atom edible subatomic particles by eric muller introduction:

With the discovery of protons, neutrons, and electrons, physicists could put forth a ….. • a periodic table (see below) These particles were electrically neutral and called neutrons. These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. Atoms with the same number of protons but different numbers of neutrons 2. Today we know over 200.
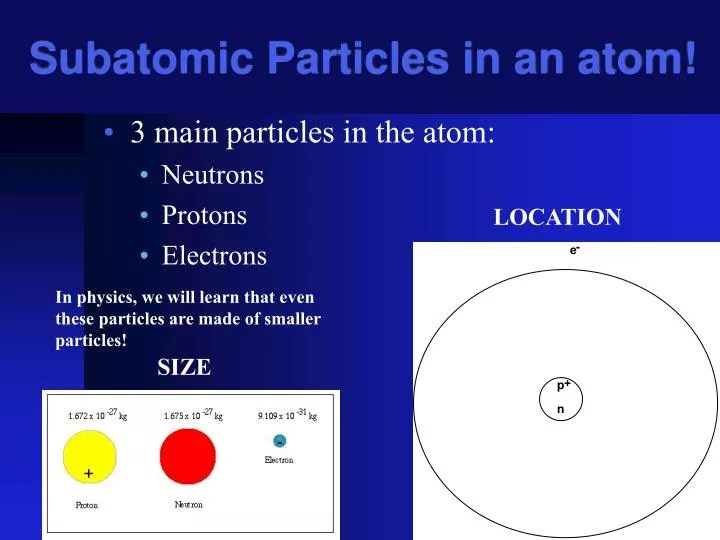
A positively charged subatomic particle 3. A subatomic particle with no charge s. The numbers of subatomic particles in an atom … Use colored candy to represent subatomic particles and make a model of an atom (bohr model). These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. Electrons, photons, protons, neutrons, antiparticles, pions, muons, kaons, baryons, neutrinos and quarks. M and m® model of the atom edible subatomic particles by eric muller introduction: • a periodic table (see below). A negatively charged subatomic particle g.
The numbers of subatomic particles in an atom … The central part of an atom containing protons and neutrons match each item with the correct statement: A negatively charged subatomic particle g.. Subatomic particles include electrons, which are the negatively charged, almost massless particles which nevertheless account for most of the size of the atom.
A subatomic particle with no charge s... Atoms with the same number of protons but different numbers of neutrons 2. These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u. Download my free ebook 10 secrets to acing organic chemistry here: These particles emitted had the same mass as protons and the relative mass of such a particle was 1 a.m.u.
